By definition, a liposome consists of a lipid bilayer that encapsulates an aqueous phase. Liposomes are increasingly used to deliver drugs in a targeted and size-controlled manner. This is where microfluidics comes in. Indeed, the science of fluid handling systems is used for the extremely precise control of very small volumes, which is very appropriate in the generation of micro- and nanosized liposomes. In this application note, we will compare literature to results obtained with two designs of microfluidic chips for generating nanoliposomes: the herringbone micromixer and the hydrodynamic flow focusing. These microfluidic chips have received a hydrophilic coating. To generate nanoliposomes, we chose to use PLGA (Poly(Lactic-Co-Glycolic Acid) solubilized in acetonitrile. The aqueous phase consists of water and surfactant (1% PVA).
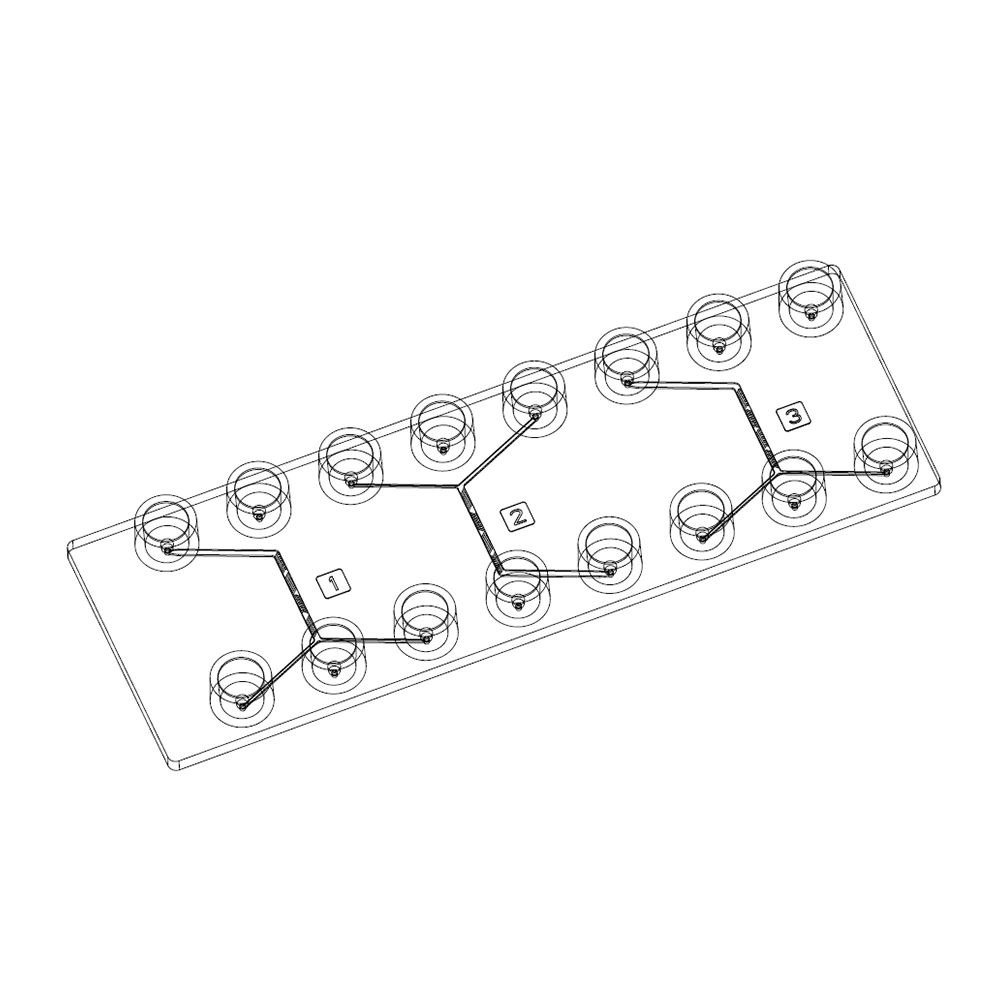
Herringbone mixer
The herringbone micromixer chip is an all-in-one solution that allows the mixing of two fluids in a microfluidic channel in a simple and efficient way. This tool is suitable for many applications such as immunoassays or PCR product mixes, but also for the generation of nanoliposomes. This type of chip can be made in various materials, including PDMS, glass and thermoplastics (PC, COC, COP, etc.).
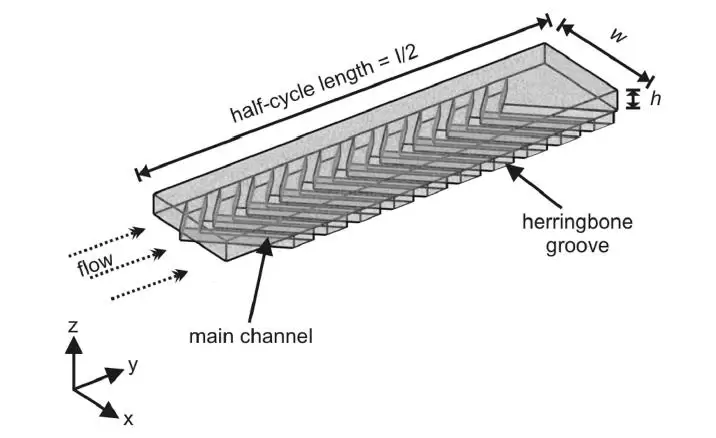
With this method, it is possible to generate nanoliposomes of a size between 100 and 200 nm, according to Roces et al. 2020, Pharmaceutics.
In practice, we generated nanoliposomes using a herringbone micromixer chip made of polycarbonate (microfluidic ChipShop, 10000019). The results indicate that we generated nanoliposomes with an average size of 247 nm ± 78.8 nm. The measurements were made by dynamic light scattering using the Zetasizer Nano ZS on 11 samples with flow rate ratios ranging from 1 to 15.
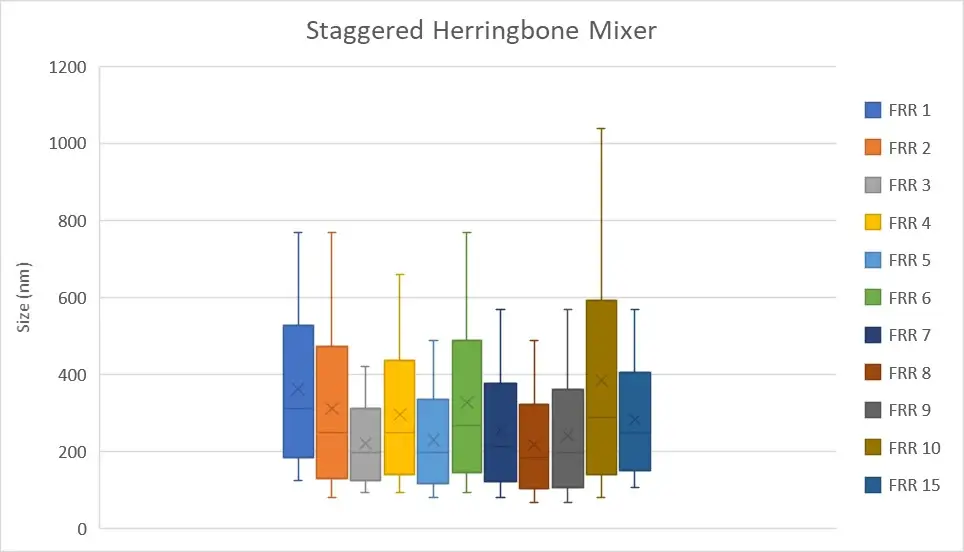
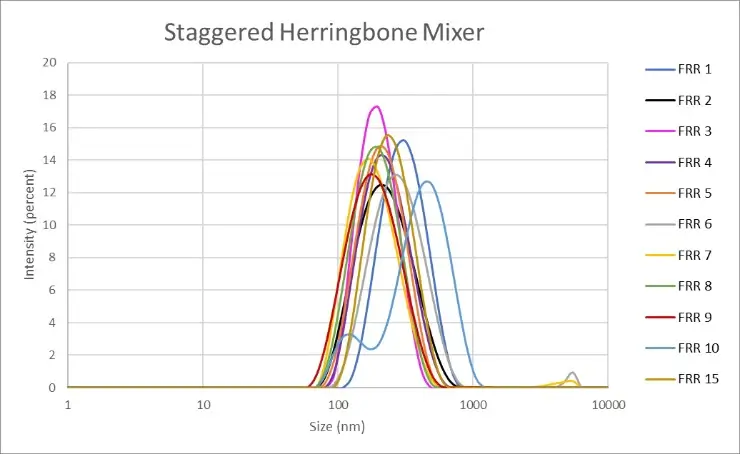
Figure 2: Measurement of the nanoliposomes diameters generated by a straggered herringbone mixer device (microfluidic ChipShop, 10000019). "FRR" stands for flow rate ratio: it corresponds to the ratio between the flow of the continuous phase and the flow of the dispersed phase. Measurements performed by Christelle Angély on a Zetasizer Nano ZS (Malvern Panalytical) of the biochemistry and biophysics platform of the Institute of Psychiatry and Neuroscience of Paris.
Hydrodynamic flow focusing
The hydrodynamic flow focusing microfluidic device allows for precise mixing of the reagents with short mixing times under laminar flow conditions, which enables the generation of, for example, monodisperse nanoparticles of adjustable size.
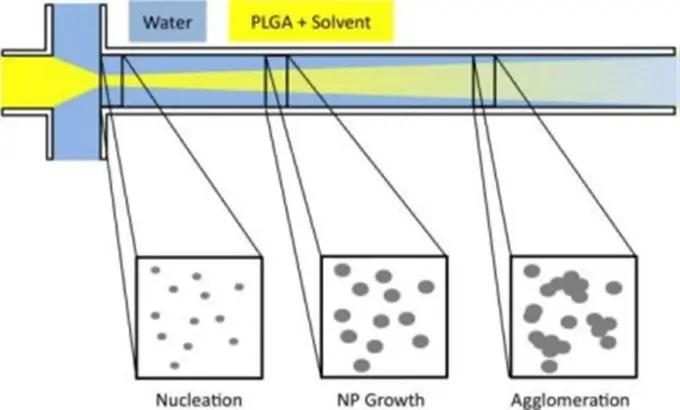
Using this flow-focusing technique, it is possible to generate nanoliposomes with a size >150 nm and usually around 200 nm. However, depending on the composition of the nanoliposomes, their size can change considerably as in the work of Karnik et al. presented in 2008. Indeed, the team is able to produce nanoparticles of 31 nm in diameter (± 1 nm) in a highly monodisperse manner.
In our case, we were able to generate nanoliposomes with an average size of 250.9 nm ± 66.8 nm using a flow-focusing chip made of polycarbonate (microfluidic ChipShop, 10000004). The measurements were made by dynamic light scattering using the Zetasizer Nano ZS on 11 samples with flow rate ratios ranging from 1 to 15.
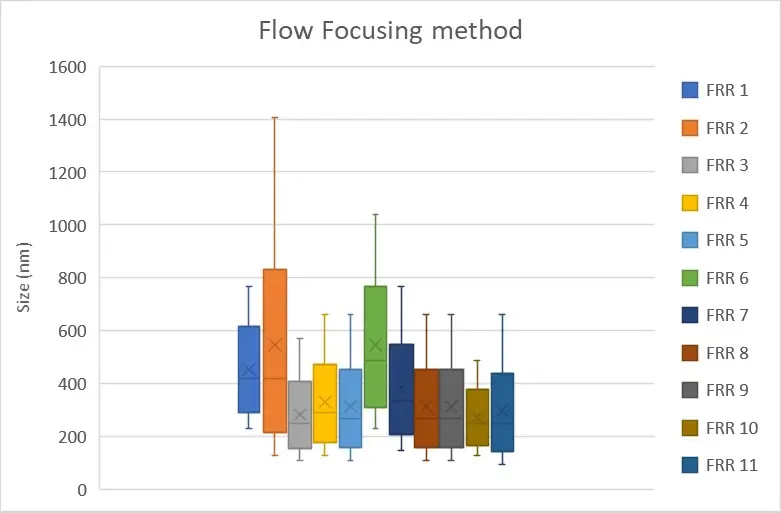
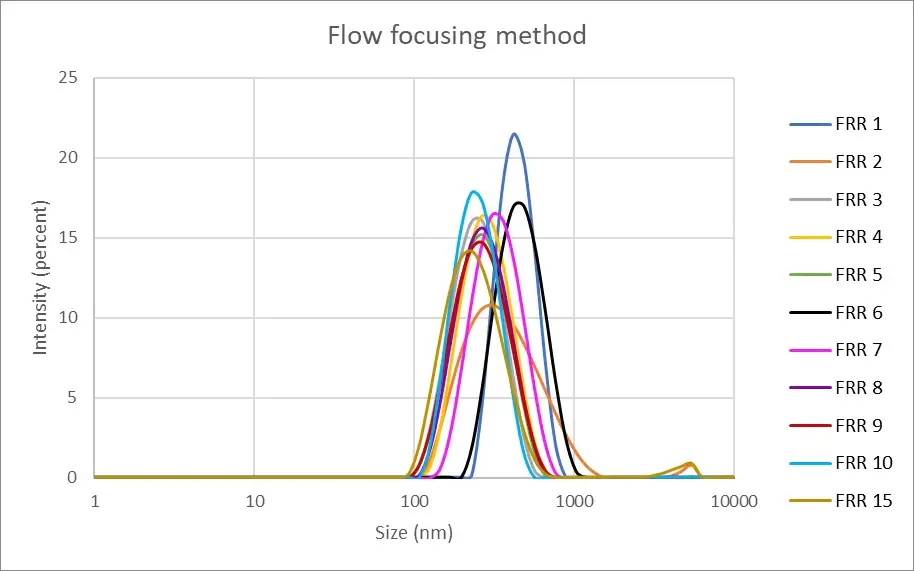
Figure 4: Measurement of the nanoliposomes diameters generated by a flow focusing device (microfluidic ChipShop, 10000004). "FRR" stands for flow rate ratio: it corresponds to the ratio between the flow of the continuous phase and the flow of the dispersed phase. Measurements performed by Christelle Angély on a Zetasizer Nano ZS (Malvern Panalytical) of the biochemistry and biophysics platform of the Institute of Psychiatry and Neuroscience of Paris.
In conclusion, the two methods presented here were able to generate nanoliposomes of similar size, i.e. about 250 nm in diameter, regardless of the flow rate ratio. The choice of the composition of the nanoliposome, whether it be the choice of lipid, solvent or aqueous phase, should therefore be taken into consideration. In addition, several well-documented techniques are effective in reducing the size of the nanoliposomes generated. For example, it is possible to perform a sonication (1 to 5 min) of the sample to reduce its size by up to 50%. Numerous publications describe filtration and/or dialysis as very efficient ways to purify nanoliposomes and thus reduce their size (from 50 to 80 nm).